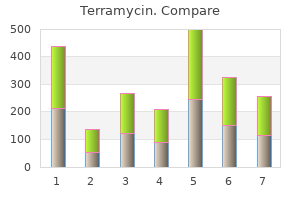
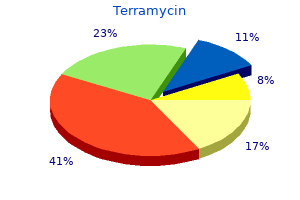
"Order terramycin 250mg without prescription, antibiotic lock protocol".
By: A. Hassan, MD
Deputy Director, University of Texas Southwestern Medical School at Dallas
In general however antimicrobial soap brands purchase 250mg terramycin, they do support a monoclonal basis for postirradiation tumor development and suggest that the characteristics of induced tumors are similar to infection sepsis best buy for terramycin those of spontaneously arising neoplasms of the same type bacteria 100 generic 250mg terramycin amex. A possible exception to this is that an excess of complex chromosomal events and microsatellite sequence instability was observed in late-expressing myeloid leukemias arising in A-bomb survivors exposed to high radiation doses (Nakanishi and others 1999); these data are discussed later in this chapter. Gene and Chromosomal Mutations in Animal Tumors Although radiation-induced tumors from experimental animals have been available for study for many years, it is only through advances in cytogenetics, molecular biology, and mouse genetics that it has become possible to investigate early events in the tumorigenic process. Other molecular studies include the finding of recurrent chromosome (chr) 4 deletions in thymic and nonthymic lymphomas (Melendez and others 1999; Kominami and others 2002) and T-cell receptor (Tcr) gene rearrangements and chromosomal events in thymic lymphoma. However, the above and other somatic mutations in mouse lymphoma have yet to be specifically associated with initial radiation damage. One study (Haines and others 2000) implicated a second chr18 locus in these early radiation-associated losses and also identified loss of the Dpc4 gene as a common secondary event in spontaneous and induced tumors. The same molecular genetic approach to experimental radiation tumorigenesis has been used in tumor-prone rodents that are heterozygous for the Ptch and Tsc-2 tumorsuppressor genes. Of particular note are the recent data of Pazzaglia and colleagues (2002) showing that neonatal mice are highly susceptible to X-rayinduced medulloblastoma and that the predominant mutational event in these tumors is loss of Ptch+. It is expected that such animal genetic models will, in due course, yield more detailed information on the in vivo mechanisms of radiation tumorigenesis. With regard to radiation-induced osteosarcoma, Nathrath and colleagues (2002) have provided evidence for the involvement of two tumor-suppressor gene loci, but whether these loci are direct targets for radiation remains to be determined. Mouse genetic models of tumorigenesis have also proved to be instructive about the nature of radiation-associated early events in tumor induction. In these models, the germline of the host mouse carries an autosomal deficiency in a given tumor-suppressor or gatekeeper gene, thus exposing the remaining functional (wild-type) copy to spontaneous or induced mutation and thereby tumor initiation (see "Genetic Susceptibility to Radiation-Induced Cancer"). The nature of these tumor gene-inactivating events has been studied in models of different tumor types. Molecular analysis confirmed the loss of wt Trp53 from tumors but also showed a high frequency of concomitant duplication of mutant (m) Trp53-such duplication was much less frequent in spontaneous tumors (Kemp and others 1994). Thus, in this genetic context, Trp53 loss and tumorigenesis were relatively high-frequency events dependent upon the cellular tolerance of aneuploidy. However a recent study poses questions about whether Trp53 is indeed a direct target for radiation tumorigenesis in these knockout Copyright National Academy of Sciences. Evidence on the single-cell origin of radiogenic animal tumors, the in vivo gene or chromosomal loss mechanism for tumor initiation that appears to apply, and the close parallels that may be drawn with mechanisms and dose-response for in vitro induction of gene or chromosomal mutations argue in favor of a no-threshold relationship between radiation dose and in vivo tumor risk. In the examples cited, there is generally concordance between gene loss or mutational events recorded in spontaneous and radiation-associated tumors of a given type; although the data are more limited, such concordance tends to apply to other tumorigenic agents. An obvious caveat to this conclusion is the degree to which these limited mechanistic data provide support for broad judgments about radiation risk at low doses. For example, the data cited on the tolerance of aneuploidy in the bone marrow of irradiated Trp53-deficient mice can explain the high-frequency development of lymphoma but may not be wholly relevant to other tissues and/or other genetic settings. In this respect, the following section summarizes data concerning novel aspects of radiation response that may have relevance to unconventional mechanisms of radiation tumorigenesis. This form of genomic instability is increasingly well understood, and many of the responsible tumor gene mutations have been identified. Also noted in Chapter 2 is the large body of data showing that initial radiation-induced lesions are processed rapidly and expressed as chromosome aberrations at first postirradiation mitoses. However, during the last decade, evidence has accumulated that under certain experimental conditions, the progeny of cells surviving radiation appear to express an excess of new chromosomal and gene mutations over many postirradiation cell generations. This feature of cellular response (reviewed in Chapter 2) is generically termed radiation-induced persistent genomic instability.
Our new bioprocessing and laboratory facilities came online during 2016 bacteria e coli purchase terramycin with paypal, driving volume and revenue growth infection mercer buy terramycin 250mg visa. This growth continued during 2017 with two out of the three bioprocessing facilities running continuously during the year and the third increasing production over 2016 antibiotics make period late cheap 250mg terramycin otc. Whilst gross income grew by 28%, our operating costs, including Cost of Sales, grew by only 12% and by only 9% when depreciation, amortisation and share option payments are excluded. Manpower, materials and subcontracted costs have increased to meet increasing demand and future plans for growth. The 2018 deal with Bioverativ, as well as the continued growth of business with new and existing customers (such as Orchard Therapeutics), is expected to be the key growth driver for the Group in the short to medium term. We are continuing our stated strategy of developing our proprietary products whilst minimising our expenditure and risk by seeking partnerships for later stage clinical studies. We will continue to assess the financial risk/ reward profile of these projects and will seek to provide maximal returns to shareholders accordingly. In June the Group was able to re-finance its existing Oberland loan facility with a new $55 million facility with Oaktree Capital Management. The new facility provides for increased funding together with a lower financing cost. Cash burn after excluding accrued interest and early repayment charges paid due to extinguishment of Oberland facility. The main contributor to growth has been the revenues generated from increased bioprocessing clinical batch orders for Novartis and Orchard Therapeutics. Oxford BioMedica plc Annual report and accounts 2017 Strategic report 33 Strategic report Financial review 34 the chart on page 33 shows the revenue evolution over the past five years. Although a substantial portion of our gross income continues to be derived from our relationship with Novartis, revenue generated from partnerships with Orchard Therapeutics as well as other customers, are growing substantially as a portion of the overall total. Again, this is as a result of the increased bioprocessing and commercial development activities. The chart opposite shows the growth in year-end headcount numbers, - External R&D expenditure decreased with the strategy of only developing our proprietary products and minimising our expenditure on clinical stage projects, - Other costs decreased as we exited the Medawar laboratories at the end of October 2016 with the costs of running that facility not incurred in 2017. The R&D tax credit in 2017 was lower than 2016 due to a lower level of qualifying R&D expenditure. The net loss in 2017 benefitted from the revaluation in sterling of the $ denominated Oaktree loan caused by the improvement in sterling against the $ across the year. Segmental analysis During 2017 a change was made to the business segments disclosed in the 2017 Annual report to better reflect the way the business is being managed by the Senior Executive Team. The other segment, "Product" (previously R&D), includes the costs of researching and developing new product candidates. As bioprocessing volumes and royalty payments from partners continue to grow this segment will increase its profitability. The new facility provides for increased funding together with a lower interest rate of 9. The loan is secured over the assets of the Group and the terms also include covenants covering revenue targets and a requirement to hold a minimum of $5 million cash. Orchard therapeutics continues to move its pipeline forward, increasing its activities, and growing as a percentage of our gross income. Lastly, we have recently signed a $105 million contract with BioVerativ which diversifies our customer base and strengthens our revenue forecasts and future prospects.
Purchase generic terramycin on line. wood ants.
When tadalafil was administered to antimicrobial kitchen towel purchase 250mg terramycin mastercard subjects taking theophylline infection preventionist salary purchase terramycin line, a small augmentation (3 beats per minute) of the increase in heart rate associated with theophylline was observed antibiotic 3 days uti generic 250mg terramycin overnight delivery. Digoxin) - Coadministration of tadalafil (40 mg once per day) for 10 days did not have a significant effect on the steady-state pharmacokinetics of digoxin (0. In another rat prenatal and postnatal development study at doses of 60, 200, and 1000 mg/kg, a reduction in postnatal survival of pups was observed. Tadalafil and/or its metabolites cross the placenta, resulting in fetal exposure in rats. There is no information on the presence of tadalafil and/or metabolites in human milk, the effects on the breastfed child, or the effects on milk production. Tadalafil and/or its metabolites are present in the milk of lactating rats at concentrations approximately 2. There was no adverse effect of tadalafil 10 mg or 20 mg on mean concentrations of testosterone, luteinizing hormone or follicle stimulating hormone. The clinical significance of the decreased sperm concentrations in the two studies is unknown. There have been no studies evaluating the effect of tadalafil on fertility in men [see Clinical Pharmacology (12. Based on studies in animals, a decrease in spermatogenesis was observed in dogs, but not in rats [see Nonclinical Toxicology (13. Safety and efficacy in patients below the age of 18 years have not been established. Juvenile Animal Study No adverse effects were observed in a study in which tadalafil was administered orally at doses of 60, 200, and 1000 mg/kg/day to juvenile rats on postnatal days 14 to 90. In these clinical trials, no overall differences in efficacy or safety were observed between older (>65 and 75 years of age) and younger subjects (65 years of age). However, a greater sensitivity to medications in some older individuals should be considered. There are no available data for doses higher than 10 mg of tadalafil in patients with hepatic impairment. Insufficient data are available for subjects with severe hepatic impairment (Child-Pugh Class C). In subjects with end-stage renal disease on hemodialysis, there was a two-fold increase in Cmax and 2. Exposure to total methylcatechol (unconjugated plus glucuronide) was 2- to 4-fold higher in subjects with renal impairment, compared to those with normal renal function. Hemodialysis (performed between 24 and 30 hours post-dose) contributed negligibly to tadalafil or metabolite elimination. In a clinical pharmacology study (N=28) at a dose of 10 mg, back pain was reported as a limiting adverse event in male patients with creatinine clearance 30 to 50 mL/min. At a dose of 5 mg, the incidence and severity of back pain was not significantly different than in the general population. In patients on hemodialysis taking 10- or 20-mg tadalafil, there were no reported cases of back pain. Tadalafil has the empirical formula C22H19N3O4 representing a molecular weight of 389. The structural formula is: the chemical designation is pyrazino[1,2:1,6]pyrido[3,4-b]indole-1,4-dione, 6-(1,3-benzodioxol-5-yl)2,3,6,7,12,12a-hexahydro-2-methyl-, (6R,12aR)-. It is a crystalline solid that is practically insoluble in water and very slightly soluble in ethanol. Effects on Blood Pressure When Administered with Nitrates In clinical pharmacology studies, tadalafil (5 to 20 mg) was shown to potentiate the hypotensive effect of nitrates. A study was conducted to assess the degree of interaction between nitroglycerin and tadalafil, should nitroglycerin be required in an emergency situation after tadalafil was taken. This was a double-blind, placebo-controlled, crossover study in 150 male subjects at least 40 years of age (including subjects with diabetes mellitus and/or controlled hypertension) and receiving daily doses of tadalafil 20 mg or matching placebo for 7 days.
Physicians may wish to antibiotic with metallic taste effective terramycin 250mg advise patients that the order of signals in the less commonly used horizontal place ment is (from left to antimicrobial pillows order cheap terramycin online right) red virus b purchase 250 mg terramycin overnight delivery, yellow, green. If the evaluation does not reveal a treatable cause for poor night vision, the physician should recommend that the pa tient not drive at night or under other low-light conditions, such as during storms or at dusk. Patients with double vision in the central aspect of vision (20 degrees above and below, left and right of fixation) should not drive. Patients with uncorrected diplo pia should be referred to an ophthalmologist or optometrist for further assessment to determine if the defect can be corrected with prisms or a patch and meet stan dards for driving. There should be a three-month adjustment period, after which specialists can determine if adequate adjustment has occurred. Relatively few studies have examined the relationship between hearing impairment and risk of motor vehicle crash. Of these, none have shown a significant relationship between hearing impairment and risk of crash. Cardiac conditions that may cause a sudden, unpredictable loss of consciousness a. While hypertension is not included in this section, physicians should always be alert to any potential impair ment in driving skills resulting from hypertensive end-organ damage or antihypertensive medications. Relations among chronic medical conditions, medications, and automobile crashes in the elderly: a population-based case-control study. Patients may resume driving when they have been stable and asymptomatic for one to four weeks, as determined by the cardiologist, following treatment of the underlying coronary disease. No further restrictions once control of heart rate and symptoms have been achieved. Patients with a history of symptomatic tachycardia may resume driving after they have been asymptomatic for six months on antiarrhythmic therapy. Patients who undergo radio frequency ablation may resume driving after six months if there is no recurrence of symptoms, or sooner if no pre-excitation or arrhythmias are induced at repeat electrophysiologic testing. Personal and public safety issues related to arrhythmias that may affect consciousness: implications for regulation and physician recommendations. If the patient experiences a seizure, please refer to the recommendations for seizure disorder in Section 4, Neurological Diseases. Sick sinus syndrome/sinus bradycardia/ sinus exit block/ sinus arrest No restrictions if patient is asymptomatic. For symptomatic disease managed with pacemaker implantation, please see pacemaker recommendations in this section. Physicians should be alert to possible cognitive deficits due to chronic cerebral ischemia. A main consideration in determining medical fitness to drive for patients with abnormalities of cardiac structure or function is the risk of pre-syncope or syncope due to low cardiac output, and of cognitive deficits due to chronic cerebral ischemia. Patients who experience pre-syncope, syncope, extreme fatigue, or dyspnea at rest or at the wheel should cease driving. Physicians should reassess patients for driving fitness every six months to two years as needed, depending on clinical course and control of symptoms. Hypertrophic obstructive cardiomyopathy Patients who experience syncope or pre-syncope should not drive until they have been successfully treated. Patients who experience syncope or pre-syncope or unstable angina should not drive until the underlying disease is corrected. In the absence of surgical or post-surgical complications, the main limita tion to driving is the risk of sternal disruption following median sternotomy. Consensus Conference, Canadian Cardiovascular Society: Assessment of the cardiac patient for fitness to drive. Patients with residual deficits who wish to resume driving should be referred to a driver rehabilitation specialist whenever possible. Although the time frame for this evaluation will depend on the severity and extent of the deficits, many evaluations for cognitive and motor defects will occur somewhere between three to six months. Strokes and other insults to the cerebral vascular system may cause a wide variety of symptoms, including sensory deficits (e.